Structure function relations of ion channels
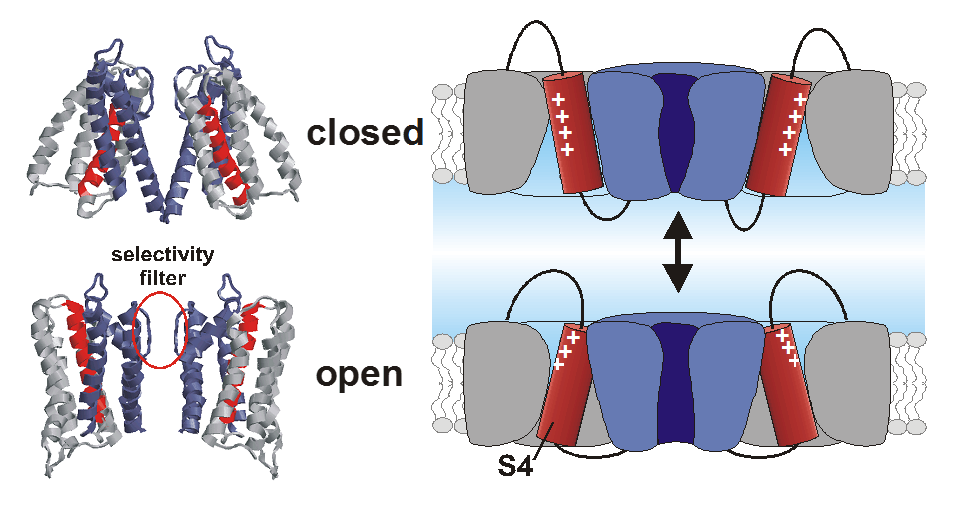
The main topic of our laboratory is to study the molecular mechanisms underlying ion channel functioning. Ion channels are integral membrane proteins that let a selective class of ions pass through the membrane along their electrochemical gradient. The channels are opened (activated) by different stimuli such as membrane potential, ligand concentration or temperature.
Structurally, ion channels are mostly multimeric proteins comprised of 3-5 subunits, and variably recruit auxiliary subunits both transmembrane and cytosolic. Ion channels are modular proteins with a central ion conducting pore and several "gating modules", peripheral domains that respond to the external stimuli.
Electromechanical coupling of voltage-gated ion channels
We are interested in the interaction between the peripheral gating domains and the central ion conducting pore, i.e. we study how the energy generated by the gating domains is transferred to the pore domain. In the case of voltage-gated ion channels, the gating domains are the voltage sensor domains (VSDs), formed by the first four transmembrane alpha-helices S1-S4. In the VSDs, the electric field is concentrated around the positive gating charges of the S4 helix leading to a conformational change upon alteration in the membrane potential. The resulting movement is transferred to the pore domain most likely via the S4-S5 linker and the C-terminal S6 (electromechanical coupling).
Studying ion chanel structure and dynamics using fluorescence spectroscopy
We are employing different fluorescence spectroscopy techniques to study the structure and function relations of ion channels.
- Studying dynamics using voltage-clamp fluorometry
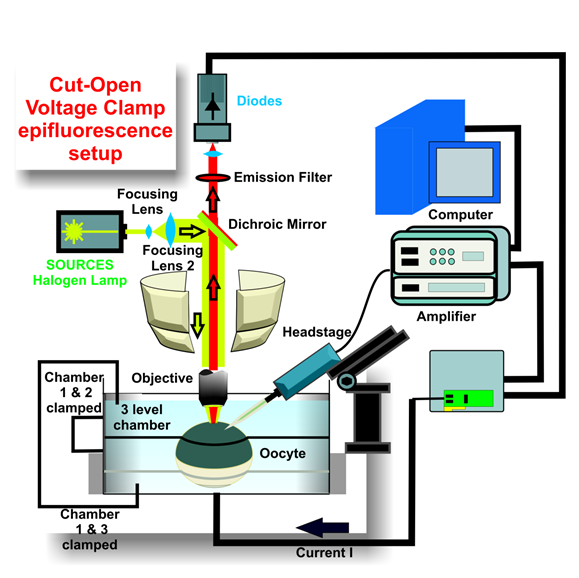
Voltage-clamp fluorometry (VCF) describes the simultaneous recording of the function (via electrophysiology) and the structural rearrangements (via fluorescence) of an electrogenic protein with temporal resolution. VCF has proven very powerful to elucidate the structure-function relations of membrane proteins, in partcular voltage-gated ion channels. The channels are expressed in Xenopus oocytes and fluorescently labeled at the position of interest mostly by reacting a thiol-reactive linker with the SH group of an accessible cysteine. Conformational changes move the fluorophores into a new environment, leading to fluorescence changes.
VCF with thiol-reactve labeling required constructs free of any accessible endogenous cysteines and could only be employed to probe externally accessible positions. The use of fluorescent unnatural amino acids (fUAA) removed these limitations (Kalstrup et al., 2013). By inserting a nonsense mutation at the location of interest and using a compatible orthogonal tRNA/tRNA-pair that recognizes the fluorescent unnatural amino acid Anap (Lee et al., 2009), we can position a fluorescent probe at any position within the protein, allowing to label also on the cytosolic side of the protein as well as buried positions.
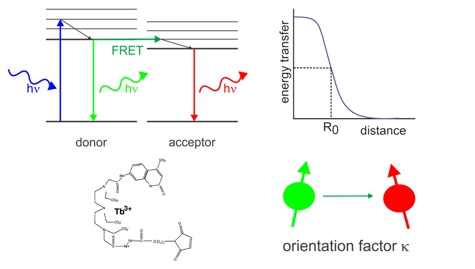 Lanthanide-based Resonance Energy Transfer (LRET)
Lanthanide-based Resonance Energy Transfer (LRET)Lanthanide-based Resonance Energy Transfer (LRET) allows to determine distances between two labeling sites with Å-resolution. LRET is a variant of Foerster resonance energy transfer (FRET) that uses a Lanthanide-chelate instead of a typical fluorophore and thereby circumvents the calculation of the dipol-dipol orientation factor responsible for much of the uncertainty in FRET-based distance calculations. The long luminescence lifetime in the ms-range also lets us determine the lifetimes with high accuracy.
The distances determined with LRET can be used as harmonic constraints in molecular dynamics simulations. For more details, please refer to Faure et al., JBC 2012 .
While ensemble fluorescence measurements give us a plethora of information, some questions cannot be answered in the ensemble average. This applies in particular whenever the key problem describes not only a heterogenous population but more specifically its internal distribution. This includes for instance
- The relative movement of ion channel subunits (Blunck et al., 2008)
- The distribution of subunits in a heteromeric protein (Groulx et al., 2011; McGuire et al., 2012). see also here.
- Dynamics of the assembly of membrane proteins or membrane protein complexes.
